|
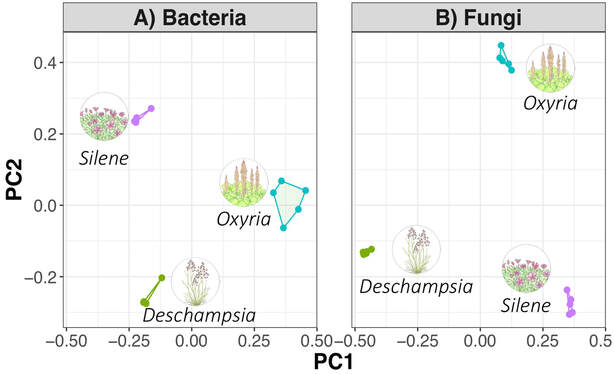
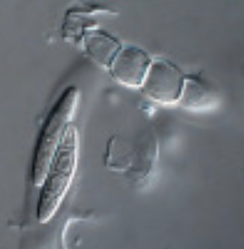
by Cliff Bueno de Mesquita This month I’ll write about another microbe from my litter addition experiment. One of the dominant fungi on/in the leaves of the alpine cushion plant Silene acaulis was Phaeosphaeria silenes-acaulis, which actually gets its name due to its presence on the Silene acaulis plant! Not only was this fungus abundant in Silene leaves, but it was not present in the other two plant species’ leaves that I studied, highlighting it’s specificity to Silene, although it has also been reported in a couple of other arctic/alpine plant species (Steinke and Hyde 1997, Tojo et al. 2013). The community composition of leaf fungi (and bacteria too) was significantly different in the three plant species I studied, and this was partially driven by the abundance of P. silenes-acaulis in Silene acaulis and not the other two species (Figure 1). Statistical analysis showed that P. silenes-acaulis contributed 14% to differences in fungal communities between Silene and the bunchgrass Deschampsia, and 31% to differences in fungal communities between Silene and the edible alpine mountain sorrel plant Oxyria. The genus Phaeosphaeria is in the phylum Ascomycota and contains saprotrophic fungi that function as decomposers. Sixteen different species in the genus have been previously reported in arctic and alpine ecosystems, including Svalbard, Norway and the Tatra Mountains in Slovakia (Tojo et al. 2013, Kozłowska et al. 2016). These fungi are adapted to these ecosystems by having a simple life cycle, forming thick and deeply pigmented walls, and having ascospores (reproductive spores produced by fungi in the Ascomycota phylum, Figure 2) with gelatinous sheaths (Leuchtmann 1987). Another species of note in the genus is Phaeosphaeria oryza, which associates with rice and is an important biological control agent of rice blast disease (Ohtake et al. 2008). As with many microbial taxa, we have now done the basic ecological work of sequencing and phylogenetic classification, but information is lacking on function. As I have done with some of my root samples, future work could attempt to isolate this fungus by placing Silene acaulis leaves on petri dishes with agar and an energy source, and then do further experiments to examine the role of the fungus either in decomposition or Silene acaulis plant health.
References: Bueno de Mesquita C.P., Schmidt S.K., Suding K.N. (2019) Species-specific plant-microbe interactions in an early successional ecosystem. Plant and Soil in review. Kozłowska M., Mułenko W., Bacigálová K, Wołczańska A., Świderska-Burek U., Pluta M. (2016) Microfungi of the Tatra Mountains. Part 7. Correction of some data from herbaria and the literature. Acta Mycologica 51(2):1081. Leuchtmann A. (1987) Phaeosphaeria in the Arctic and Alpine Zones. In: Laursen G.A., Ammirati J.F., Redhead S.A. (eds) Arctic and Alpine Mycology II. Environmental Science Research, vol 34. Springer, Boston, MA Ohtaka N., Kawamata H., Narisawa K. (2008) Suppression of rice blast using freeze-killed mycelia of biocontrol fungal candidate MKP5111B. Journal of General Plant Pathology 74: 101-108 Quaedvlieg W., Verkley G.J., Shin H.D., Barreto R.W., Alfenas A.C., Swart W.J., Groenewald J.Z., Crous P.W. (2013) Sizing up Septoria. Studies in Mycology 75: 307-390. Tojo M., Masumoto S., Hoshino T. (2013) Phytopathogenic Fungi and Fungal-Like Microbes in Svalbard. In: Imai R., Yoshida M., Matsumoto N. (eds) Plant and Microbe Adaptations to Cold in a Changing World. Springer, New York, NY Steinke T.D., Hyde K.D. (1997) Phaeosphaeria capensis sp. nov. from Avicennia marina in South Africa. Mycoscience 38: 101-103.
0 Comments
Leave a Reply. |
AuthorVarious lab members contribute to the MoM Blog Archives
October 2023
Categories |

 RSS Feed
RSS Feed
