|
by Cliff Bueno de Mesquita and Lara Vimercati Happy new year from all of us here at the Alpine (and Arctic and Antarctic) Microbial Observatory! For our first post of 2020 we will keep posting about some of the organisms in our freeze-thaw, water and nutrient amendment experiment on soils from Volcán Llullaillaco, Chile, led by Dr. Lara Vimercati. By the third water addition to microcosms receiving only water and no nutrients, we observed a significant increase in relative abundance of an OTU that was a 100% match with Neochlorosarcina negevensis, a member of the Chlorophyceae. N. negevensis is an alga commonly found in biological soil crusts of drylands (Büdel et al. 2016). Layers of algae within the Chlorophyceae have been identified as being the closest phototrophs to the soil surface within gypsum deposits at low elevations in the Atacama Desert (Wierzchos et al. 2015). Many Chlorophyceae have the capability to synthesize high amounts of complex molecules, such as carotenoids, that enhance cell resistance under unfavorable environmental conditions including excess light, UV, water and nutrition stress (Hanagata and Dubinsky, 1999; Orosa et al., 2000, 2001). Other strategies for survival in harsh conditions include the development of dormant stages or avoidance through motility (Pushkareva et al. 2016). Previous work has shown that in arid environments, high relative humidity is enough to induce metabolic activity of some algae (Palmer and Friedmann 1990); however, it is unclear whether these microalgae may be functioning in situ once water becomes available following a snowmelt event. More work will be needed to determine if Neochlorosarcina sp. is a functional phototroph in this environment. 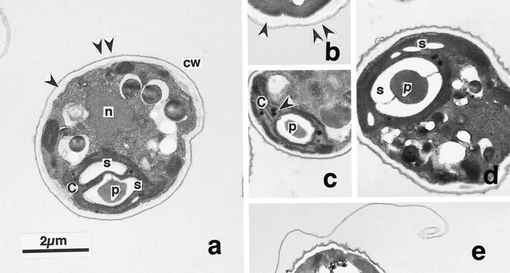 Figure 1. Transmission electron micrographs of 0-day old green cells of the alga Scenedesmus komarekii (Chlorophyceae, Chlorophyta). a. Section of vegetative cell showing distribution of major organ-elles. Arrowhead and double arrowhead show inner and outer layers of cell wall, respectively. b. Cell wall consisting of inner (arrowhead)and outer (double arrowhead) layers. c. Plastoglobuli (arrowhead) distributed in the chloroplast. d. Biplate and small starch grains inthe chloroplast. e. Curled outer layer of a parent cell wall. Abbreviations: c, chloroplast; cw, cell wall; n, nucleus; p, pyrenoid; s, starchgrain. From Hanagata and Dubinsky 1999. Of broader significance, this is the first report of phototrophs on the high elevation slopes of Volcán Llullaillaco not associated with semi-permanent water structures such as fumaroles or penitentes (Costello et al. 2009, Solon et al. 2018, Vimercati et al. 2019b). This ecosystem was previously thought to be completely heterotrophic or chemolithotrophic dependent (Lynch et al. 2014). It was believed that in this harsh ecosystem, energy entered through the system through aeolian deposited carbon sources and chemolithotrophic fixation of inorganic compounds; however, these new findings suggest that it may also enter the system via photosynthesis. However, it is important to note that this work was conducted using soils from 5100 m.a.s.l. whereas the studies mentioned above were conducted on soils from between 5300 and 6300 m.a.s.l. Therefore, more work is needed to determine if there is an elevational limit to phototrophic life not associated with penitentes or fumaroles on these volcanoes.
References: Costello, E.K., S.R.P. Halloy, S.C. Reed, P. Sowell, S.K. Schmidt. 2009. Fumarole-supported islands of biodiversity within a hyperarid, high-elevation landscape on Socompa Volcano, Puna de Atacama, Andes. Appl. Environ. Microbiol. 75: 735-747. Hanagata, N., and Dubinsky, Z., 1999. Secondary carotenoiaccumulation in Scenedesmus komarekii (Chlorophyceae, Chlorophyta). Journal of Phycology 35(5): 960-966. Lynch, R.C., Darcy, J.L., Kane, N.C., Nemergut, D.R. and Schmidt, S.K., 2014. Metagenomic evidence for metabolism of trace atmospheric gases by high-elevation desert Actinobacteria. Frontiers in microbiology, 5, p.698. Orosa, M., Torres, E., Fidalgo, P., and Abalde, J., 2000. Production and analysis of secondary carotenoids in green algae. Journal of Applied Phycology 12: 553-556. Orosa, M., Franqueira, D., Cid, A., and Abalde, J., 2001. Carotenoid accumulation in Haematococcus pluvialis in mixotrophic growth. Biotechnology Letters 23: 373-378. Palmer, R.J. and Friedmann, E.I., 1990. Water relations and photosynthesis in the cryptoendolithic microbial habitat of hot and cold deserts. Microbial ecology, 19(1), pp.111-118. Pushkareva, E., Johansen, J.R. and Elster, J., 2016. A review of the ecology, ecophysiology and biodiversity of microalgae in Arctic soil crusts. Polar Biology, 39(12), pp.2227-2240. Solon, A.J., Vimercati, L., Darcy, J.L., Arán, P., Porazinska, D., Dorador, C., Farías, M.E. and Schmidt, S.K., 2018. Microbial Communities of High-Elevation Fumaroles, Penitentes, and Dry Tephra “Soils” of the Puna de Atacama Volcanic Zone. Microbial Ecology, pp.1-12. Vimercati L., Solon, A.J., Krinsky, A., Arán, P., Porazinska, D.L., Darcy, J.L., Dorador, C., Schmidt, S.K. 2019. Nieves penitentes are a new habitat for snow algae in one of the most extreme high-elevation environments on Earth. Arct. Antarct. Alp. Res. 51(1): 190-200
0 Comments
|
AuthorVarious lab members contribute to the MoM Blog Archives
October 2023
Categories |

 RSS Feed
RSS Feed
