Microbial Succession
Microbial Community Assembly and Ecosystem Succession
This project addresses hypotheses regarding the relationship between microbial community assembly, environmental parameters, and ecosystem functioning during primary succession (following glacial retreat) and secondary succession (following forest fires). High-throughput community analyses and metagenomics are employed to explore the factors controlling how microbial communities develop (microbial assembly processes) and the relative roles of environmental (niche) and random (neutral) controls in structuring microbial communities (Nemergut et al. 2013). A wide array of biogeochemical processes are monitored along the chronosequences in order to link community assembly with ecosystem functions. Research sites are in Colorado, Alaska and Perú. Recent publications from this work highlight the unexpected importance of nutrient limitations (especially phosphorus limitation) in controlling both community assembly and ecosystem functions during the microbial stages of ecological succession in both Perú (Darcy et al. 2018, Schmidt et al. 2012) and Alaska (Darcy & Schmidt 2016, Schmidt et al. 2016).
One of the driving force behind this project was Professor Diana Nemergut, who passed away on December 31st, 2015. Funding was provided by NSF grants DEB-0922267 (Links between Soil Biogeochemistry and Microbial Community Dynamics Along Recently Deglaciated Chronosequences), and DEB-1258160 (“Collaborative Research: Relative Controls of Niche vs. Neutral Microbial Community Assembly Processes Over Ecosystem Function Post-Disturbance").
This project addresses hypotheses regarding the relationship between microbial community assembly, environmental parameters, and ecosystem functioning during primary succession (following glacial retreat) and secondary succession (following forest fires). High-throughput community analyses and metagenomics are employed to explore the factors controlling how microbial communities develop (microbial assembly processes) and the relative roles of environmental (niche) and random (neutral) controls in structuring microbial communities (Nemergut et al. 2013). A wide array of biogeochemical processes are monitored along the chronosequences in order to link community assembly with ecosystem functions. Research sites are in Colorado, Alaska and Perú. Recent publications from this work highlight the unexpected importance of nutrient limitations (especially phosphorus limitation) in controlling both community assembly and ecosystem functions during the microbial stages of ecological succession in both Perú (Darcy et al. 2018, Schmidt et al. 2012) and Alaska (Darcy & Schmidt 2016, Schmidt et al. 2016).
One of the driving force behind this project was Professor Diana Nemergut, who passed away on December 31st, 2015. Funding was provided by NSF grants DEB-0922267 (Links between Soil Biogeochemistry and Microbial Community Dynamics Along Recently Deglaciated Chronosequences), and DEB-1258160 (“Collaborative Research: Relative Controls of Niche vs. Neutral Microbial Community Assembly Processes Over Ecosystem Function Post-Disturbance").
|
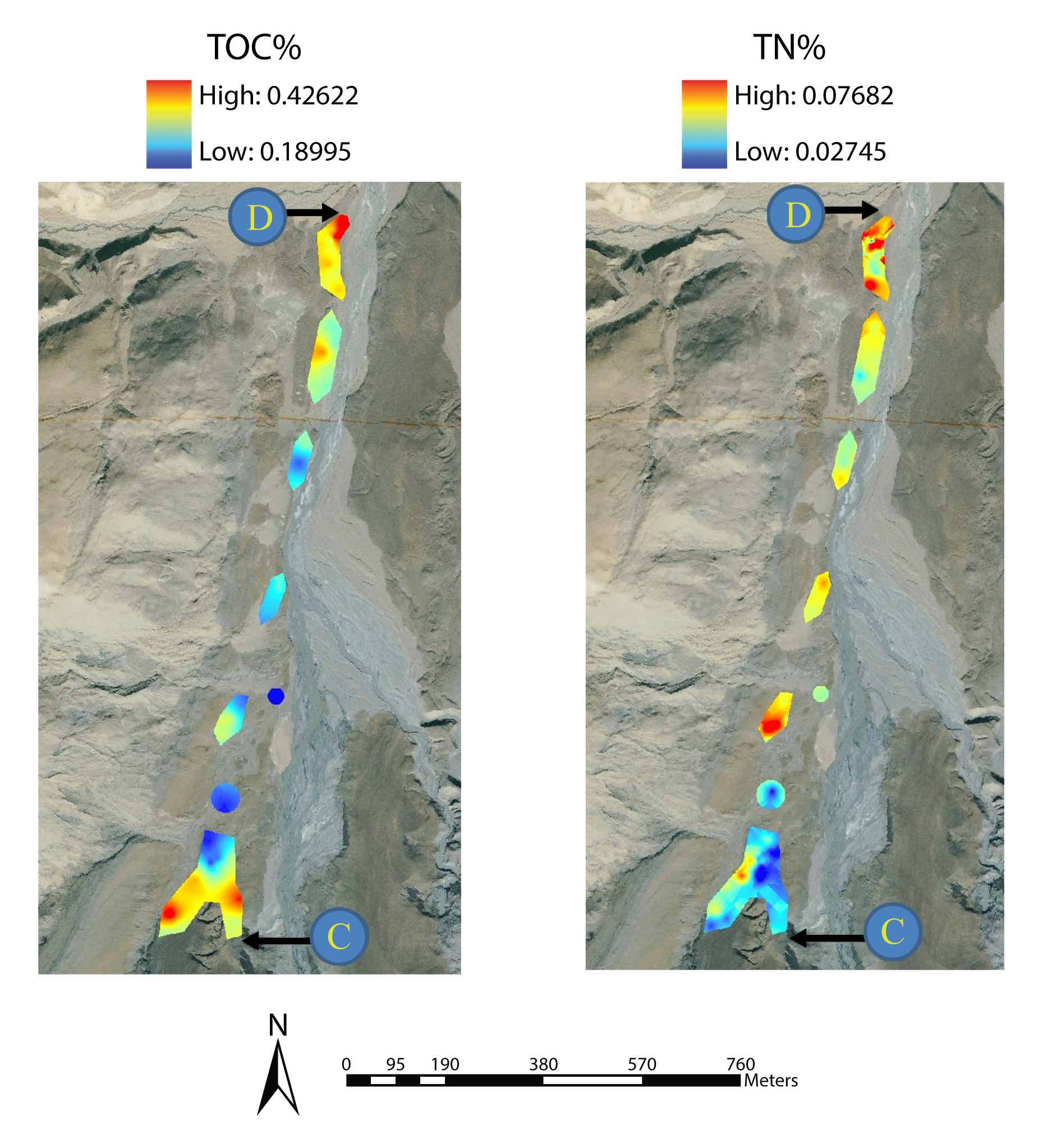
Heat maps showing the landscape patterns of total organic carbon (TOC) and Total organic nitrogen (TON) across the MF Toklat Glacier chronosequence, Denali National Park. Note the hotspots of TOC near the glacier terminus (C) and at the farthest distance from the glacier terminus (D). In contrast TON hotspots were more randomly arrayed along the chronosequence. This is Figure 7 from: Ecosystems 19: 1164–1177 (2016). [Reprint] This figure was created by Andrew King, by overlaying landscape patterns in the parameters onto an aerial photo of the field site |
Relevant publications (reverse chronological order):
Darcy J.L, et al. 2018. Phosphorus, not nitrogen, limits plants and microbial primary producersfollowing glacial retreat. Science Adv 4: eaaq0942
Knelman JE et al. Rapid Shifts in Soil Nutrients and Decomposition Enzyme Activity in Early Succession Following Forest Fire. Forests 8: doi:10.3390/f8090347
Castle S.C. et al. 2017. Nutrient limitation of soil microbial activity during the earliest stages of ecosystem development. Oecologia 185: 513-524.
Schmidt S.K. et al. 2016. Biogeochemical stoichiometry reveals P limitation across the post-glacial landscape of Denali National Park. Ecosystems 19: 1164–1177 (2016). [Reprint]
Nemergut D.R. et al. 2016. Decreases in average bacterial community rRNA operon copy number during succession. ISME Journal 10: 1147-1156. ISME Journal 10: 1147-1156 [Reprint]
Darcy J.L., S.K. Schmidt. 2016. Nutrient limitation of microbial phototrophs on a debris-covered glacier. Soil Biol. Biochem. 95: 156-165 [Reprint]
Castle, S.C. et al. 2016. Biogeochemical drivers of microbial community convergence across actively retreating glaciers. Soil Biology Biochemistry. 101: 74-84. [Reprint]
Schmidt S.K. et al. 2014. Do bacterial and fungal communities assemble differently during primary succession? Molecular Ecology. 23: 254-258 [Enhanced Reprint]
Knelman J.E. et al. 2014. Nutrient addition dramatically accelerates microbial community succession. PLoS ONE 9(7): e102609 [Reprint]
Ferrenberg, S. et al. 2013. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME Journal 7: 1102-1111 [Reprint]
Nemergut D.R., et al. 2013. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 77: 342-356. [Reprint]
Schmidt S.K. et al. 2012. A simple method for determining limiting nutrients for photosynthetic crusts. Plant Ecology and Diversity 5: 513-519. [Reprint]
Schmidt S.K. et al.. 2011. Estimating phosphorus availability for microbial growth across a recently de-glaciated landscape in the high Andes of Perú. Geoderma 163: 135-140. [Reprint]
Sattin, S.R. et al. 2009. Functional shifts in unvegetated, perhumid, recently-deglaciated soils do not correlate with shifts in soil bacterial community composition. J. Microbiol. 47: 673-681 [Reprint]
Schmidt S.K., et al. 2008. The earliest stages of ecosystem succession in high-elevation (5000 meters above sea level), recently de-glaciated soils. Proc. Roy. Soc. B 275: 2793-2802. [Reprint]
Nemergut, D.R. et al. 2007. Microbial community succession in unvegetated, recently-deglaciated soils. Microbial Ecology 53: 110-122. [Reprint]
Darcy J.L, et al. 2018. Phosphorus, not nitrogen, limits plants and microbial primary producersfollowing glacial retreat. Science Adv 4: eaaq0942
Knelman JE et al. Rapid Shifts in Soil Nutrients and Decomposition Enzyme Activity in Early Succession Following Forest Fire. Forests 8: doi:10.3390/f8090347
Castle S.C. et al. 2017. Nutrient limitation of soil microbial activity during the earliest stages of ecosystem development. Oecologia 185: 513-524.
Schmidt S.K. et al. 2016. Biogeochemical stoichiometry reveals P limitation across the post-glacial landscape of Denali National Park. Ecosystems 19: 1164–1177 (2016). [Reprint]
Nemergut D.R. et al. 2016. Decreases in average bacterial community rRNA operon copy number during succession. ISME Journal 10: 1147-1156. ISME Journal 10: 1147-1156 [Reprint]
Darcy J.L., S.K. Schmidt. 2016. Nutrient limitation of microbial phototrophs on a debris-covered glacier. Soil Biol. Biochem. 95: 156-165 [Reprint]
Castle, S.C. et al. 2016. Biogeochemical drivers of microbial community convergence across actively retreating glaciers. Soil Biology Biochemistry. 101: 74-84. [Reprint]
Schmidt S.K. et al. 2014. Do bacterial and fungal communities assemble differently during primary succession? Molecular Ecology. 23: 254-258 [Enhanced Reprint]
Knelman J.E. et al. 2014. Nutrient addition dramatically accelerates microbial community succession. PLoS ONE 9(7): e102609 [Reprint]
Ferrenberg, S. et al. 2013. Changes in assembly processes in soil bacterial communities following a wildfire disturbance. ISME Journal 7: 1102-1111 [Reprint]
Nemergut D.R., et al. 2013. Patterns and Processes of Microbial Community Assembly. Microbiol. Mol. Biol. Rev. 77: 342-356. [Reprint]
Schmidt S.K. et al. 2012. A simple method for determining limiting nutrients for photosynthetic crusts. Plant Ecology and Diversity 5: 513-519. [Reprint]
Schmidt S.K. et al.. 2011. Estimating phosphorus availability for microbial growth across a recently de-glaciated landscape in the high Andes of Perú. Geoderma 163: 135-140. [Reprint]
Sattin, S.R. et al. 2009. Functional shifts in unvegetated, perhumid, recently-deglaciated soils do not correlate with shifts in soil bacterial community composition. J. Microbiol. 47: 673-681 [Reprint]
Schmidt S.K., et al. 2008. The earliest stages of ecosystem succession in high-elevation (5000 meters above sea level), recently de-glaciated soils. Proc. Roy. Soc. B 275: 2793-2802. [Reprint]
Nemergut, D.R. et al. 2007. Microbial community succession in unvegetated, recently-deglaciated soils. Microbial Ecology 53: 110-122. [Reprint]