|
by Eli Gendron Dinoflagellates are an extremely morphologically and ecologically diverse group of organisms. Dinoflagellates are mainly characterized by their two dissimilar flagella (whiplike appendages used for swimming), with a transverse flagella that beats to the side of the cell and a conventional flagella that beasts posteriorly, and a cell covering called an amphiesma or cortex (Figure 1a). Many dinoflagellates, such as Karenia brevis in particular, are capable of filling multiple ecological roles as they are capable of auxotrophic heterotrophy (meaning they can utilize both organic and inorganic compounds as food), as well as also possessing chloroplasts allowing them to perform photosynthesis as well (Figure 1b) [Vargo 2009]. Karenia brevis is most well-known for its role in harmful algae blooms (HABs) and is one of the primary organisms responsible for the phenomena known as ‘red tides’ seen off the Florida coast [Ross et al. 2010]. One of the reasons HABs of Karenia brevis are so disruptive to ecosystems is due to their ability to produce brevetoxins. Brevetoxins are neurotoxins produced by dinoflagellates such as Karenia brevis, and when their populations bloom during HABs, the concentration of these toxins can reach harmful and potentially deadly levels resulting in fish kills, coral death, and even human illness [Baden 1989]. Currently, it is not currently known for certain why Karenia brevis and other dinoflagellates produce brevetoxins. One of the more recent debates revolves around the hypothesis that brevetoxins are produced in response to osmotic stress felt by these organisms [Errera & Campbell 2011; Sunda et al. 2013]. 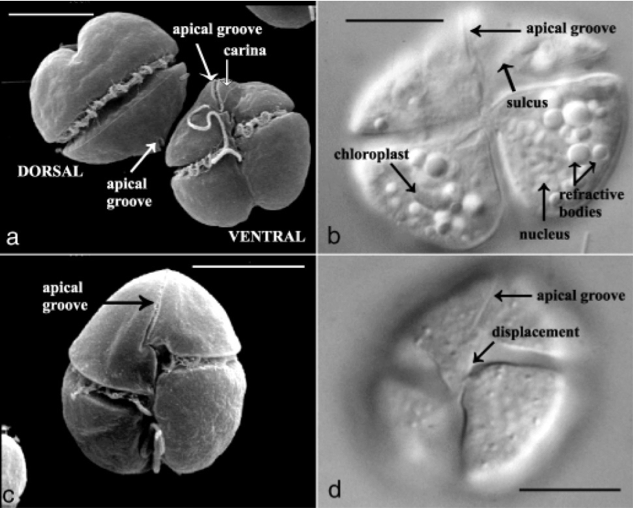 Figure 1. Karenia brevis. Scanning election micrograph (a + c) of dorsal and ventral views highlights the two flagella and characteristic apical groove. Light microscopy (b + d) of Karenia brevis ventral view highlighting organelles (particularly chloroplasts). Scale bars, 10um. Figure adapted from [Haywood et al. 2004]. References:
Baden, D. G. (1989). Brevetoxins: unique polyether dinoflagellate toxins. The FASEB journal, 3(7), 1807-1817. Errera, R. M., & Campbell, L. (2011). Osmotic stress triggers toxin production by the dinoflagellate Karenia brevis. Proceedings of the National Academy of Sciences, 108(26), 10597-10601. Haywood, A. J., Steidinger, K. A., Truby, E. W., Bergquist, P. R., Bergquist, P. L., Adamson, J., & Mackenzie, L. (2004). COMPARATIVE MORPHOLOGY AND MOLECULAR PHYLOGENETIC ANALYSIS OF THREE NEW SPECIES OF THE GENUS KARENIA (DINOPHYCEAE) FROM NEW ZEALAND 1. Journal of Phycology, 40(1), 165-179. Ross, C., Ritson-Williams, R., Pierce, R., Bullington, J. B., Henry, M., & Paul, V. J. (2010). Effects of the Florida red tide dinoflagellate, Karenia brevis, on oxidative stress and metamorphosis of larvae of the coral Porites astreoides. Harmful Algae, 9(2), 173-179. Sunda, W. G., Burleson, C., Hardison, D. R., Morey, J. S., Wang, Z., Wolny, J., ... & Van Dolah, F. M. (2013). Osmotic stress does not trigger brevetoxin production in the dinoflagellate Karenia brevis. Proceedings of the National Academy of Sciences, 110(25), 10223-10228. Vargo, G. A. (2009). A brief summary of the physiology and ecology of Karenia brevis Davis (G. Hansen and Moestrup comb. nov.) red tides on the West Florida Shelf and of hypotheses posed for their initiation, growth, maintenance, and termination. Harmful Algae, 8(4), 573-584.
1 Comment
|
AuthorVarious lab members contribute to the MoM Blog Archives
October 2023
Categories |

 RSS Feed
RSS Feed
